The photoelectric effect is the phenomenon that electrons jump out when light is shined on the surface of a metal. However, there were three inexplicable facts when it was discovered.
Three mysterious facts that could not be explained
- There was a threshold frequency of light at which the photoelectric effect occurs, not the intensity.
- At that frequency, increasing the light intensity would cause the number of electrons to be popped up exactly proportionately.
- The maximum value of the kinetic energy of the protruding electron depends only on the frequency of the light.
The threshold frequency is the value of frequency that the photoelectric effect occurs. It seemed easy to explain above mysteries, but at that time, it wasn’t so straightforward because physicists thought that “light is a wave”. According to the wave nature of light, the above phenomena seemed impossible to understand.
Why is phenomenon 1 strange?
Suppose electrons pick up the energy of light to be popped out of the metal. If the intensity of light is increased, the electrons will jump out accordingly. However, if the light is lower than the threshold frequency, electrons will never jump out from the metal at all. It is always true no matter how much the light intensity is increased.
Why is phenomenon 2 strange?
This was evident that the amounts of electrons jumping out were proportional to the intensity of light at the threshold frequency. However, it is strange if light is a wave as the continuous property. In other words, it cannot be explained that certain electrons protrude in exactly proportion to the intensity of light. If light had only the wave nature, electrons would have various energies with a certain statistical range.
Why is phenomenon 3 strange?
As mentioned earlier, increasing the light intensity means increasing the energy of light, but the electrons that jump out depend on the frequency of the light regardless of its energy. This is difficult to explain if light has only the wave property.
What is the problem of light as waves?
It is well-known in experiments that light travels as a transverse wave, and we can explain many phenomena with the theory of electromagnetic waves.
However, there was also a phenomenon that energy of light is not continuous, but energy of an integer multiple of a certain value. This value is called Planck’s constant, and this was proved by his experiment. In fact, this discrete energy is turned out to be the frequency multiplied by Planck’s constant.
What is Einstein’s light quantum hypothesis?
From the discovery from Planck, Einstein set up a light quantum hypothesis and attempted to explain the mysteries of the photoelectric effect. The series of hypothesis were as follows:
- Light is a flow of particles with energy \(E=h\nu \), which is called a photon.
- One photon flips out precisely one electron.
- The law of conservation of energy in the photoelectric effect is expressed as \(\frac{1}{2}mv^2=h\nu – W\).
Now, we can explain the photoelectric effect by using energy conservation law expressed in C. A photon with energy \(h\nu\) hands over all e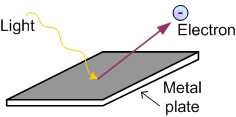 nergy to one free electron in the metal. The electrons that receive this energy leave the metal with the energy of W, and then they fly away with kinetic energy of \(\frac{1}{2}mv^2\).
nergy to one free electron in the metal. The electrons that receive this energy leave the metal with the energy of W, and then they fly away with kinetic energy of \(\frac{1}{2}mv^2\).
Would you explain three mysterious facts by using a light quantum hypothesis?
The threshold frequency produces the energy that the electrons in the metal are about to jump out. In other words, when the kinetic energy of electrons is zero in the law of conservation of energy, the energy with the threshold frequency is equal to the work function.
For electrons that protrude accurately in proportion to the light intensity, it is explained by the property that all the energy of photons is handed over to electrons, which is the most important fact of the hypothesis.
The third mystery can obviously be explained from the relational expression of the light frequency and energy.
Photo credit: Sam-H-A via Visual Hunt / CC BY
